Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
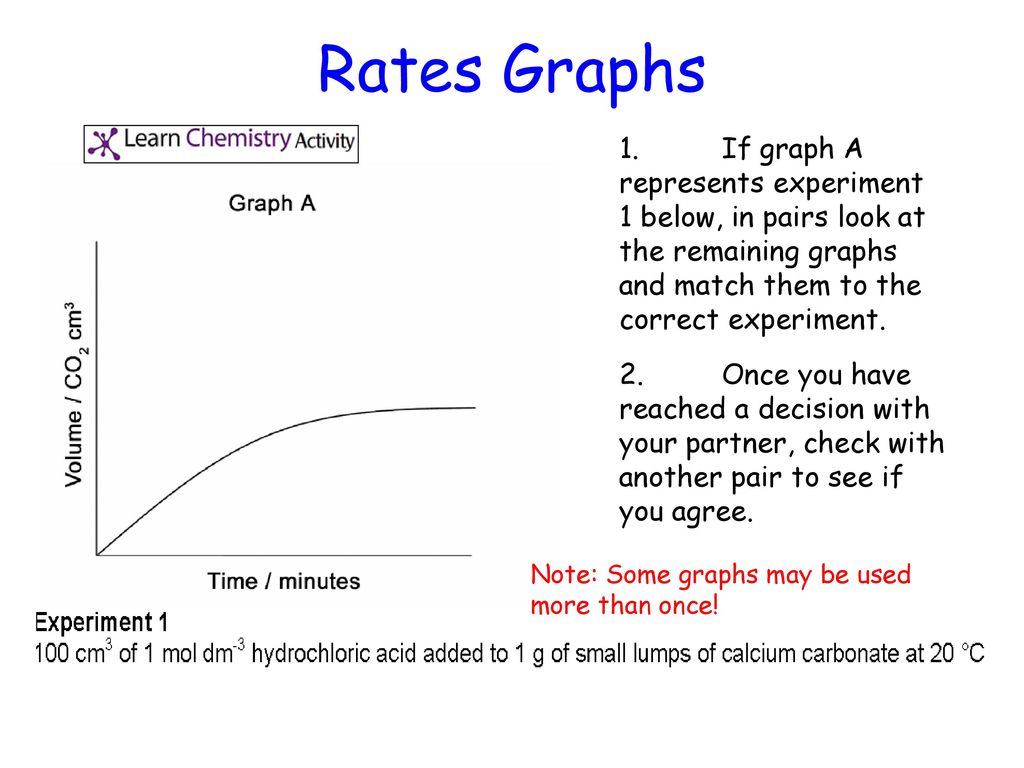
Marble chips and hydrochloric acid graph.
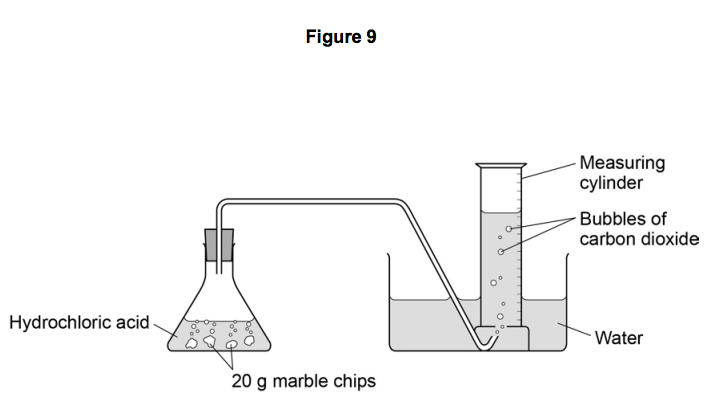
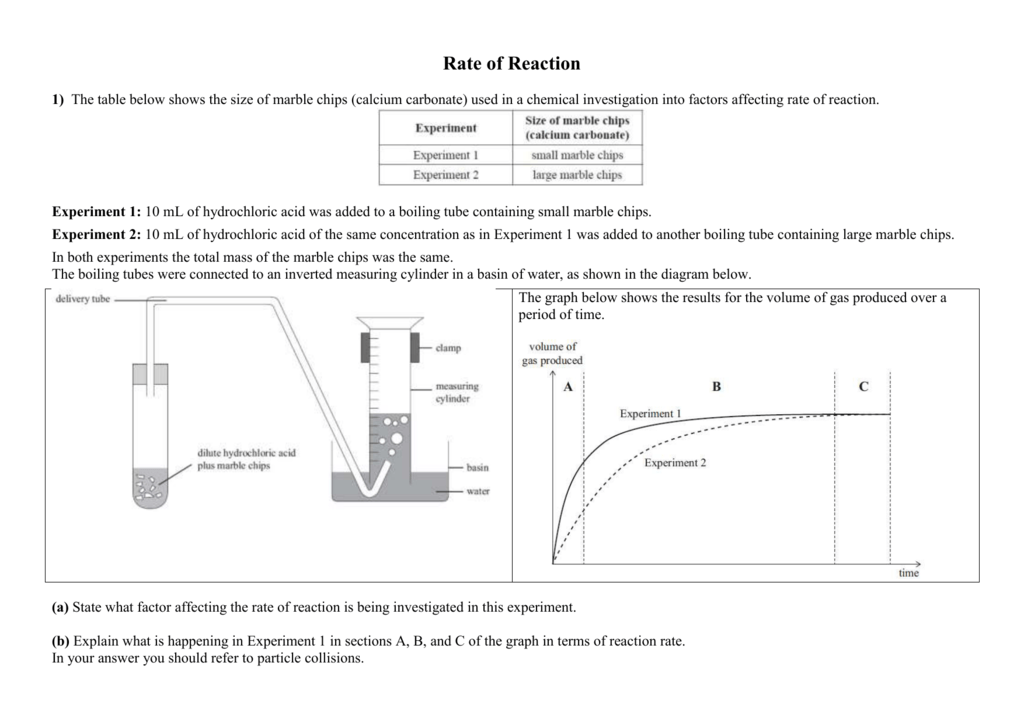
In the reaction between calcium carbonate marble chips and hydrochloric acid we can use the apparatus below to find the rate of reaction.
Marble chips placed onto pieces of paper.
0 5 g of magnesium ribbon and 0 5 g of magnesium powder are reacted with identical samples of hydrochloric acid.
Conical flask delivery tube bung measuring cylinder x 2 water trough water stopwatch marble.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
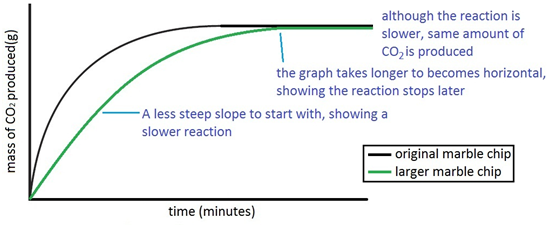
The rate of this reaction can be changed by changing the size of the marble chips.
Measuring the rate of loss of a gaseous product.
Diagram plan the equipment i will be using for this experiment will be as follows.
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
C reaction of marble chips and acid.
Measured out 1ml of water in a 10ml measuring cylinder and placed into the test tube labelled 2.
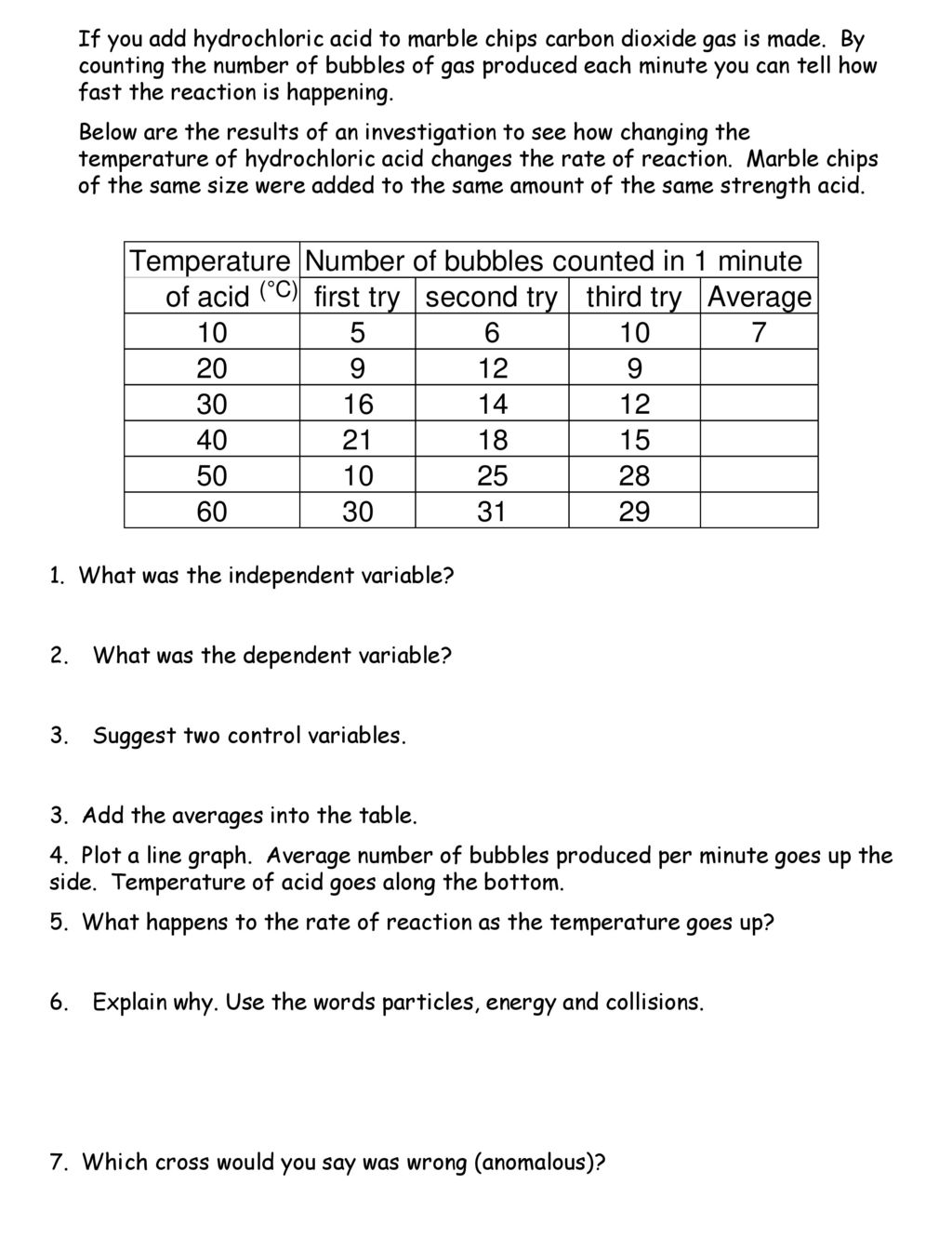
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
An investigation into how changing one variable influences the rate of reaction between marble chips and dilute hydrochloric acid planning section when dilute hydrochloric acid reacts with marble chips the following reactions occurs.
A stand to hold up the measuring cylinder.
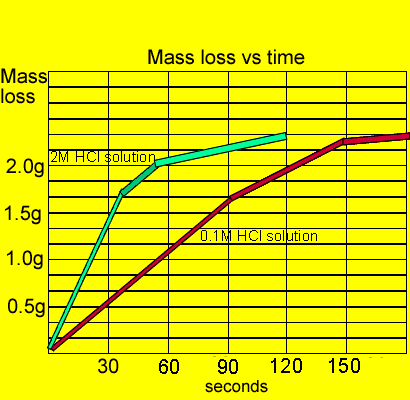
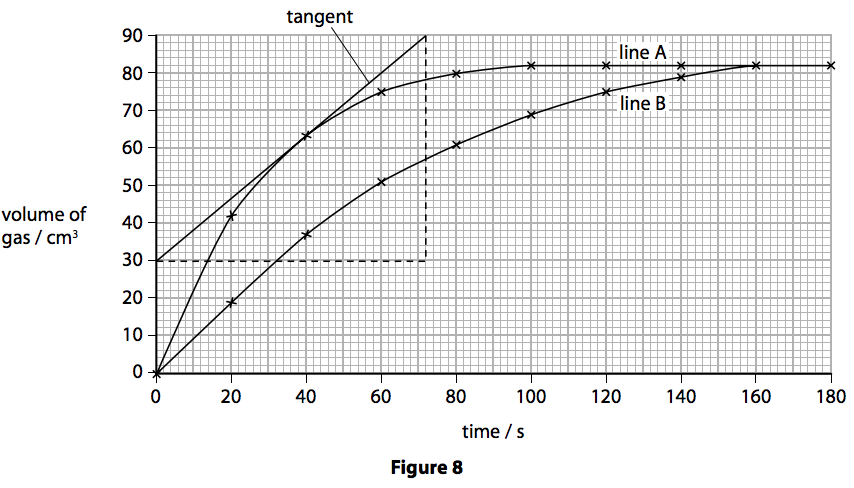
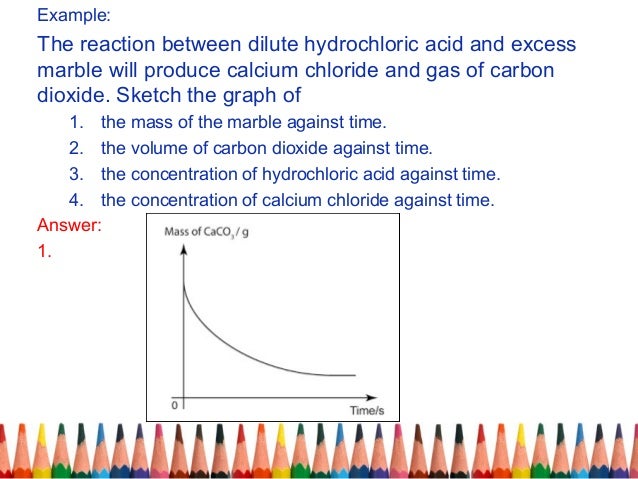
This is because as time goes on the volume of the gas evolved does not change.
Place 40cm 3 of hydrochloric acid in an conical flask.
Task my task is to measure the rate of reaction between marble chips caco 3 and hydrochloric acid 2 hcl.
The curve on the graph goes flat when the reaction is complete.
A tube to connect the conical flask to the measuring cylinder.
Plugged in scientific scales and weighed out 1g of marble chips for each test tube.
The variables that i shall be changing will be the concentration of hydrochloric acid and water.
Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method.
Measured 5ml of hydrochloric acid in the 10ml measuring cylinders and placed into each beaker separately.
A chemistry investigation to look at the rates of reaction between marble chips and hydrochloric acid.
Caco3 2hcl h2o co2 this is the reaction we will be investigating.